Planning SEEG using automated lesion detection
One‐third of children with epilepsy are medication‐resistant. In children with a focal seizure onset zone (SOZ), stereoelectroencephalography (sEEG) can be used to delineate the SOZ in complex patients. This involves electrodes being implanted into the brain to record brain activity. Currently, electrode placement is a clinical decision but planning SEEG can be challenging and time-consuming. In half of the patients selected for sEEG, the MRI scan looks normal which makes planning where to implant electrodes difficult.
In this study published in Epilepsia in 2020 we aimed to evaluate the feasibility and potential benefits of using deep learning on structural magnetic resonance imaging (MRI) to plan implantation of electrodes for stereoelectroencephalography (sEEG) in pediatric patients with drug-resistant epilepsy.
The study trained a neural network classifier to identify lesions in MRI data from 34 patients with known cortical dysplasias and 20 healthy controls.
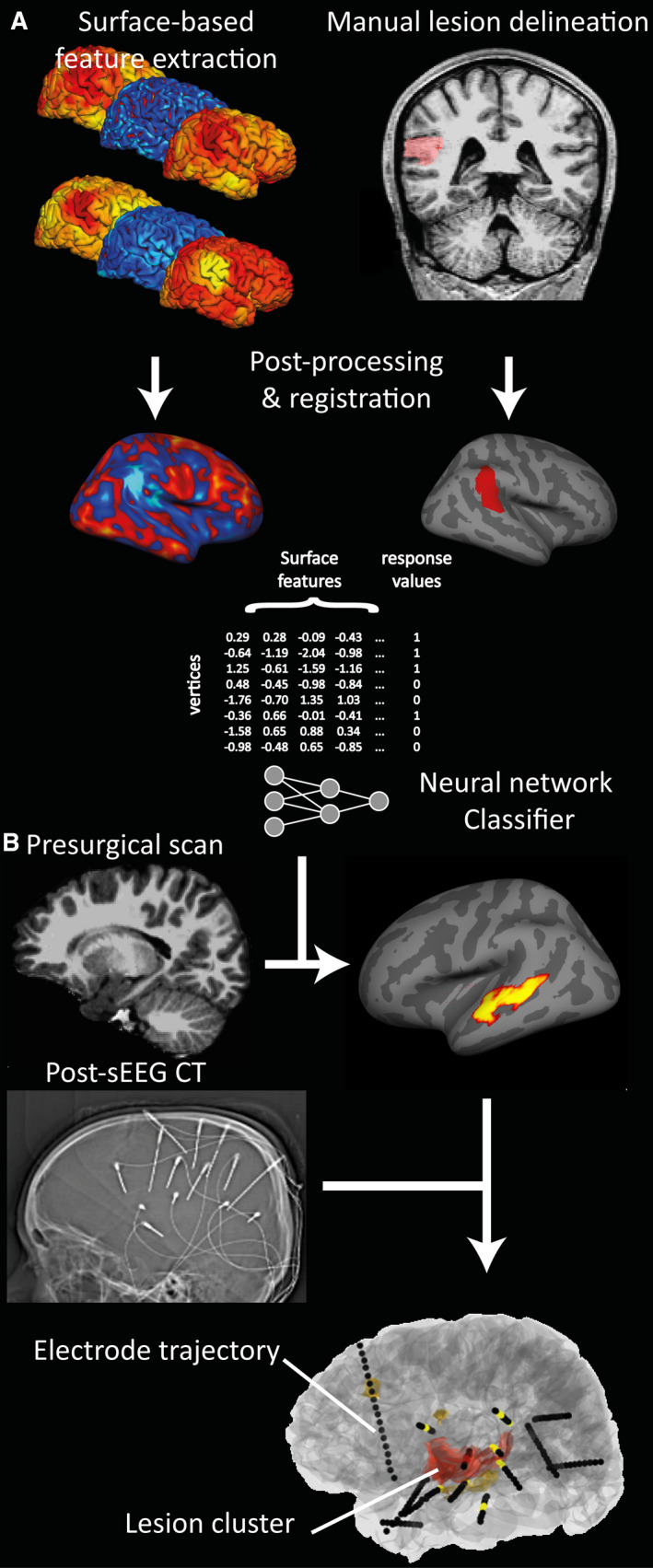
The classifier was able to detect lesions with a sensitivity of 74% in patients with known lesions and had a specificity of 100% in healthy controls. In 34 patients who underwent SEEG, we then assessed whether the seizure onset zone (SOZ) overlapped with the classifier output.
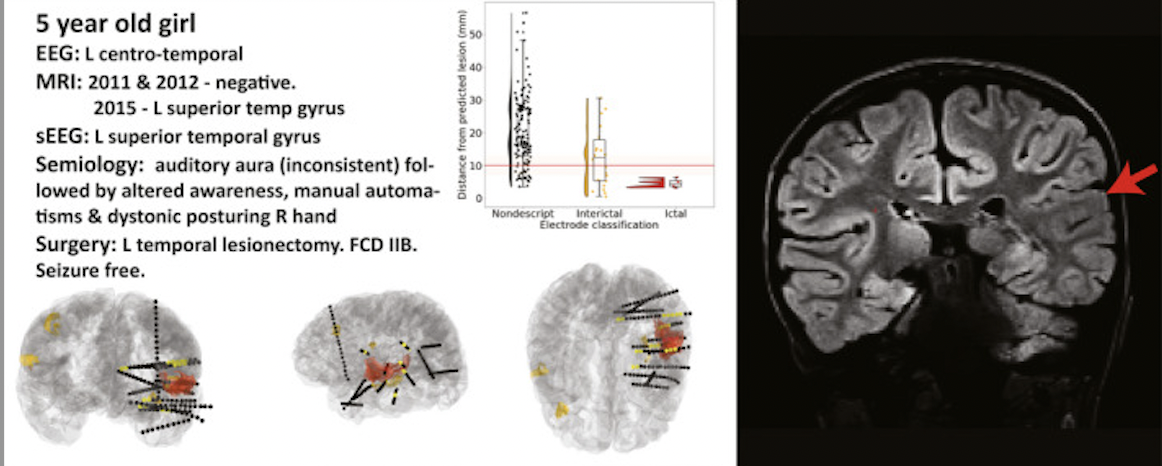
We found that in 62% of the patients with focal SOZs and 86% of histopathologically confirmed FCDs the results were concordant!
This suggested that incorporating deep-learning-based MRI analysis could be a useful tool for planning sEEG implantations.
One limitation of the study was its retrospective nature, which meant that further research was needed to determine the effectiveness of the automated method in a prospective study.
However, the study provided a framework for using automated lesion detection to plan optimal electrode trajectories and the results supported the prospective evaluation of this approach in future studies. This work led TO the MAST clinical trial!